Therapy with CAR-T Cells for Lymphoma and Leukemia
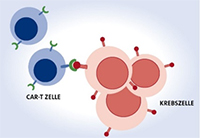
In the treatment of uncontrolled types of lymphoma and leukemia, CAR-T cell therapy offers an innovative therapeutic approach with new chances of recovery. Due to its complexity, its performance is limited to a few clinics that have extensive experience in the transplantation of cell preparations. The Medical Center – University of Freiburg is a certified center for carrying out CAR-T cell therapy.
The immune system gives the body numerous weapons to render cancer cells harmless. Sometimes, however, the immune system does not recognize the degenerated cells. In certain cases, the immune system can now be given a helping hand.
The novel gene therapy against cancer was used for the first time at the Medical Center – University of Freiburg in 2019 - with great success. The patient suffered from diffuse large cell lymphoma, the most common form of lymph node cancer, which, if left untreated, quickly leads to death. Cancer cells reappeared very quickly in the 44-year-old patient despite intensive therapy for her lymph gland tumor. As part of a so-called CAR T-cell therapy, the patient's defense cells, the so-called T-lymphocytes, were genetically modified in the laboratory so that they can recognize and fight the cancer cells. The therapy worked very well - after the use of the CAR-T cells, no more cancer cells could be detected in the patient.
As part of the therapy, T cells are removed from the patient and genetically reprogrammed in such a way that they have CAR structures on their cell surface. The resulting CAR-T cells can be multiplied millions of times in the laboratory, a process that takes about 3-4 weeks. This new "Chimeric Antigen Receptor" (CAR for short) on the surface of the T lymphocytes fits like a lock and key to structures on the tumor surface. The patient then receives his individually manufactured CAR-T cell therapy with an infusion. The modified cells will then specifically destroy the tumor cells. This therapy can be particularly effective because the CAR-T cells continue to multiply in the body for weeks, circulate tirelessly through organs and tissues and repeatedly destroy cancer cells on direct contact without being damaged themselves. Above all, the surface marker CD19 on lymphoma/leukemia cells has so far served as a point of attack.
Before the CAR-T cells are returned, chemotherapy is usually carried out to reduce the number of tumor cells in the body.
The collection of the patient's own cells, the return of the T-cell product, the clinical monitoring of the use and the management of side effects as well as the necessary aftercare of our patients is an interdisciplinary service of several clinical departments. In order to be able to identify and treat possible side effects of the therapy in good time, a hospital stay of around two weeks is necessary after the cells have been returned. This is followed by further outpatient check-ups.
The use of the therapy has so far been approved for the following indications:
- Refractory or relapsed aggressive diffuse large B-cell non-Hodgkin's lymphoma (DLBCL) (after at least two or more systemic therapies) (Tisagenlecleucel / Kymriah® and Axicabtagene Ciloleucel / Yescarta®)
- Primary refractory or relapsed acute lymphocytic leukemia (ALL) up to the age of 25 (Tisagenlecleucel / Kymriah®)
- Mantle cell lymphoma (Brexucabtagen / Tecartus®)
- Multiple myeloma (idecaptagen vicleucel / ABECMA®)
In addition to clinical treatment, there are intensive research activities with the aim of significantly expanding the areas of application for CAR-T cells in the future, including those for the treatment of solid tumors.
Back